 Hypoglycemia during oral glucose tolerance test among post–bariatric surgery pregnant patients: incidence and perinatal significance - ScienceDirect
Hypoglycemia during oral glucose tolerance test among post–bariatric surgery pregnant patients: incidence and perinatal significance - ScienceDirectMedical Case ReportsIn this PageCase Report ← Open AccessKevin Stuart, Annmarie Field, Jessie Raju, Sudarshan Ramachandran, "Postprandial Reactive Hypoglycaemia: Varying Presentation Patterns on Extended Glucose Tolerance Tests and Possible Therapeutic Approaches", Case Reports in Medicine, vol. 2013, Article ID 273957, 5 pages https://doi.org/10.1155/2013/273957Postprandial Reactive hypoglicemia: Presentation patterns on Enlarged Glucose tolerance Tests and possible therapeutic approachesSudarshan Ramachandran1Department of Clinical Biochemistry, Hospital of Good Hope, Heart of England Foundation NHS Trust, Rectory Road, Sutton Coldfield, Birmingham2 B75RR It is our practice to conduct tests of tolerance to extended glucose (eGTTs) or mixed food tests in these patients. We describe two patients who experienced hypoglycemia symptoms early and late during EGTT. The patient who experienced early symptoms, unlike the patient who presented late symptoms, did not possess any characteristic of metabolic syndrome. Based on clinical symptoms, glucose, insulin and free fatty acid (FFA) levels, we speculate on possible mechanisms that could have taken into account each of their presentation patterns. Then we speak of low Glycaemic index diet that will be the main pillar of management.1. IntroductionReactive hypoglycemia is often considered in patients who develop sympathetic or neuroglycotic symptoms in the postprandial state. Before reaching this diagnosis, it is essential that hypoglycemia associated with symptoms be demonstrated (). It has been suggested that the hypoglycemia (glucose less than 3.9 mmol/L or 70 mg/dL) while symptomatic and symptom relief after the normalization of glucose levels should replace the tolerance test of the extended cracks (eGTT). However, this may pose practical problems, as patients need laboratory blood to be performed when it is symptomatic for a reliable diagnosis. In view of this, we have been our practices to perform a first-line eGTT or a mixed food test (in case the patient can identify a particular meal or food with symptoms) with insulin and FFA measurements at the beginning of symptoms. Insulin and FFA measurements in our opinion facilitate the diagnosis of postprandial reactive hypoglycemia on a physiological basis [, ]. There has been a considerable debate on the level of glucose that defines hypoglycemia, and this has ranged between 2.2 mmol/L and 4.0 mmol/L []. The results of the EGTT carried out in our metabolic research unit have shown different clinical and biochemical patterns that require an understanding of the relationship between glucose and factors that influence it as insulin levels, insulin resistance and counterregulation measures. We have observed symptomatic hypoglycemia both early and late during eGTT. We present a couple of patients showing possible reactive hypoglycemia at different time points during the EGTT and then speculate on possible causes. This will be followed by possible actions that could be taken, focusing mainly on dietary measures.2. MethodsPatients with possible postprandial reactive hypoglycemia are often referred to the endocrine or metabolic clinics at Good Hope Hospital and are investigated in the metabolic research unit after clinical evaluation. EGTT is not recommended in patients with intercurrent disease, dumping syndrome or posturgery due to difficulty in interpreting results. It is performed following a minimum of 10 hours of fasting with only permitted water, preceded by 3 days or more of normal diet and activity. Polycal (113 mL containing 75 g of anhydric glucose, composed of up to 200 mL of water) is given orally, followed by another 100 mL of water. Blood samples (for glucose, insulin, c-peptide and free fatty acids) are performed at 0 minutes and 30 minutes intervals for 4 hours and also at the point of the patient's symptoms. Research may require discontinued if the patient is overly symptomatic and requires rescue. All samples are analyzed for glucose with insulin fatty acids, C peptides and free fats estimated in case of low blood sugar (≤3.5 mmol/L), hypoglycemic symptoms or are requested retrospectively when considered useful for diagnosis. Abnormal results are discussed at a multidisciplinary team meeting and future management and follow-up are mapped. Patients are then seen in outpatient clinics, discussed results and possible treatments discussed with proper follow-up. Glucose was measured using an enzymatic Hexokinase/G-6-PDH essay on the Abbott Architect C system, the CV essay is 2.2%. FFA was estimated at the Birmingham Children's Hospital using a colorimetric test on the Olympus 640 analyzer. Insulin was measured at the Peptide SAS Hormone Center, the Royal Surrey Hospital, Guilford, using the Iso ELISA Mercody Insulin.3. Case History We present 2 patients with hypoglycemia symptoms during EGTT at different time points and describe their clinical characteristics, recovery and biochemical changes. Patient A, a 70-year-old woman (weight: 67 kg; BMI: 24.2) had experienced dizziness and collapse for 2 years. She did not possess any of the characteristics of metabolic syndrome [, ]. A blood glucose concentration of 2.4 mmol/L had been detected while the symptomatic in primary care, and had been referred to the metabolic clinic. There was no family history of diabetes. Direct interrogation suggested an association with meals, especially those containing large quantities of carbohydrates. It was decided to carry out an eGTT. The results of the relevant biochemistry are presented in table (a) and in figure . During the test, it became symptomatic (neuroglycopic symptoms; lightheading, but without clinical evidence of sympathetic counterregulation; blood pressure and heart rate remained at 117/74 mmHg and 65 bpm, resp.) between 100 and 120 minutes without evidence of clinical improvement. There seemed to be no improvement after 130 minutes and the test was abandoned after discussion with the patient. It was given fast glucose power tablets and quickly recovered while under close medical supervision.(a) (min)Glucose (mmol/L)Insulin (pmol/L)FFA (μmol/L)04.6324973011.91760 6011.92920 906.2640(a) Time (min)Glucose (mmol/L)Insulin Results were discussed and an association between insulin levels and blood sugar was observed. The observed pattern seemed to fit into a reactive pattern, although hypoglycemia occurs early. High levels of insulin were considered to prevent physiological counterregulation; therefore, the recovery of the patient was compromised. At this point, we decided to repeat the eGTT, but using half the glucose content to determine whether a different clinical, biochemical and recovery pattern was performed. Although this modified EGTT was not conventional and may not be useful in determining the diagnosis, it was decided that it could produce important information compared to the original eGTT. In addition, it might be useful to see if the smaller glucose load would lead to less severe symptoms. The results of the modified eGTT are presented in table (b). Although the patient returned to symptomatic after 120 minutes began to recover without medical intervention with blood sugar increasing to 3.8 mmol/L after 150 minutes. FFA levels increased online with blood sugars suggesting a physiological response to hypoglycemia. Patient B, a 52-year-old man (weight: 118.2 kg; BMI: 38) had experienced severe spellings a few hours after a meal. Although it was not diabetic, it was classified as having metabolic syndrome (central weight distribution, hypertension, total cholesterol: 4.6 mmol/L; HDL-cholesterol: 0.8 mmol/L; triglycerides: 3.9 mmol/L). Due to postprandial symptoms an EGTT was performed and the results of the corresponding biochemical test are presented in Table and Figure . It became symptomatic (synomas neuroglucopenos: dizziness and blurred vision) after 180 min and the test stopped at 200 minutes due to lack of recovery, and were given quick action glucose energy tablets. Time (min)Glucose (mmol/L)Insulin (pmol/L)FFA (μmol/L)05.8 3012.79706506016.833204309013.645502011208.017101191506.9 651804.71360862103.5920124Time (min)Glucose (mmol/L)Insulin130606200 Both patients experienced symptoms due to hypoglycemia at different times after the glucose load. Patients A showed no characteristic of metabolic syndrome in contrast to patient B. Blood glucose quickly increased in both patients within 30 minutes; in patient A, it stopped increasing at that time, while in the insulin-resistant patient B, it continued to increase. Plasma insulin levels also increased in both patients, although patterns were different as seen in Figure . In patient A, insulin concentration of plasma increased earlier with peak levels observed after 60 minutes (table a), Figure ). This was followed by a rapid decrease in blood glucose. The maximum concentration of insulin was of less magnitude after half of the glucose load (table b)) with the improvement of symptoms. On the other hand, patient B showed a greater increase in insulin concentration with maximum levels occurring after 90 minutes. The decrease in blood glucose also took place later. 4. DiscussionReactive hypoglycemia is a phenomenon that can be affected by the exaggerated release of insulin and insulin resistance [, ]. The degree of abnormality of the above factors can contribute to the different hypoglycemia patterns and physiological response observed in our patients. Insulin levels increased after the glucose load in patient A and were followed by a rapid decrease in glucose levels leading to symptoms. Since this patient did not demonstrate metabolic syndrome, increased insulin sensitivity could have led to a rapid decrease in glucose. Insulin levels, although the decrease was perhaps sufficiently high during hypoglycemia to suppress the counter-regulatory response that could have led to low glucose levels. The corresponding insulin levels were much lower when the glucose load was reduced and may have allowed physiological recovery. Gluconeogenesis as a recovery mechanism is suggested by the growing levels of FFA. Improved recovery during EGTT with reduced glucose load may suggest that patient A could be treated with a diet aimed at reducing insulin response. Patients B showed higher levels of fasting insulin than patient A probably due to the underlying resistance to insulin. The reduction of glucose was much more gradual, perhaps again due to the insulin action that was overwhelmed by insulin resistance. He suffered from symptoms to a higher concentration of glucose compared to patient A. Insulin levels remain high at this time and may have contributed to the prolonged symptoms leading to the cessation of the test. The primary mechanism of patient A, which did not show insulin resistance characteristics, could have been an exaggerated and rapid increase in insulin release. The rapid absorption of glucose could also have been a factor. Finally, factors that influence insulin and glucago release should also be considered, such as increases and defects in the insulin-release mechanism itself. Patient B, instead, showed blood glucose and insulin levels during the EGTT more according to insulin resistance. The cell input of glucose is through glucose (GLUT) transport proteins that are insulin-independent or insulin-dependent [–]. GLUT 1–3 proteins are ubiquitous and permanently located in the cell surface membrane acting independently of insulin depending on the glucose concentration. Insulin promotes the movement of glucose in adipose tissue and skeletal muscle by recruiting GLUT 4 in these tissues to the plasma membrane []. GLUT 2 is the dominant channel in both the liver and pancreatic cells. The release of insulin involves alteration in the electrical activity of cell ions channels and in the cellular secretive function regulated by the soluble factor -etilmalimide-sensible that activates protein receptor proteins (SNARE). Glucose absorption by the cells increases cell production ATP which leads to an increase in the ATP ratio: ADP that in turn closes the ATP sensitive channels leading to depolarization of the membrane []. This results in the opening of Ca2+ closed voltage channels that lead to the fusion of insulin-containing granules with the plasma membrane, a step regulated by SNARE proteins []. The secretion of insulin mediated by glucose is biphasic that consists of an immediate first phase with a limited mobilization of readily available pools, followed by a second phase with greater use of the reserve pool []. SNARE proteins are involved in both processes. Insulin released binds to the insulin receptor in most of the body tissues, which leads to the initiation of the receptor's tyrosine kinase activity, which in turn allows the insertion of the GLUT 4 stored vesicular channels into the cell wall that regulates the glucose influx [–]. It has been shown that a dysfunctional link between insulin and GLUT 4 target contributes to insulin resistance [, ]. The pathogenesis of insulin resistance syndrome is multifactorial. It can reduce the oxidation of glucose, which could result in insulin secretion with deficiencies and the elimination of intracellular glucose after the delayed cell entry of glucose and the resulting hyperglycemia. Late reactive hypoglyemias as part of insulin resistance syndrome can be caused by delayed insulin secretion and therefore delayed GLUT 4 insertion []. The delayed insertion means that the GLUT 1–3 insulin-independent intake ratio has increased thus leaving a lower proportion for GLUT 4 to handle. Despite this smaller proportion, hyperinsulinemia continues to recruit more GLUT 4 inappropriately at a later stage where hyperglycemia is already approaching normoglycemia and causes rapid glucose in cells that result in hypoglycemia. Early reactive hypoglycemia may be the function of an exaggerated incretin effect []. The effect of incretin explains the phenomenon of oral glucose as a more powerful stimulus for the release of insulin than the parenteral glucose of an equal concentration. This is mediated in part by the intestinal hormones of the peptide-1 (LP-1) and the insulinotropic polypeptide dependent on glucose (GIP) and could result in excess of insulin exocytosis and thus the early regulation of GLUT 4 channels and subsequent hypoglycemia. In addition to insulin release, GLP-1 suppresses glucago and leaves the patient unable to respond to early hypoglycemia. Finally, greater oral glucose loads lead to a growing incretin effect that, in turn, can result in a more severe reactive hypoglycemia []. There was considerable benefit when both patients understood that hypoglycemia was the probable reason for their symptoms. However, it was very important that management be adapted to the biochemical pattern observed during eGTT. Both patients were considered to benefit from a smaller and more regular food intake, consisting of low-glucaemic (GI) carbohydrates. The decrease in the response to insulin, as expected, would lead to symptomatic improvement. Currently, both patients have been advised accordingly and are experiencing some benefit. Similarly, we would consider acarbosa as a second line in both patients to decrease glucose absorption. We have previously initiated a patient that demonstrates the late hypoglymic pattern in the sitagliptin (from 25 mg and cautiously increases the dose with patient consent) due to its possible delay in gastric emptiedness. We recognize that the increase in endogenous LPG-1 after a gliptin could lead to even greater insulin levels that worsen the symptoms; therefore, careful follow-up of this patient began. We would not consider this approach in patients who exhibit early postprandial hypoglycemia as an exaggerated GLP-1 response that can be causative. We would have some concerns about metformin as it could reduce physiological response to hypoglycemia, despite the potential to improve insulin sensitivity. Therefore, the treatment pillar currently remains focused on reducing the glycemic load and a low GI diet and will describe it later. GI is a measure of the effects of carbohydrates on blood sugar levels. GI estimates how each gram of available carbohydrates consumed (fiber of less total carbon carbohydrates) raises the level of blood glucose of an individual []. Foods with carbohydrates that quickly break down during digestion and quickly release glucose into the bloodstream tend to be high IG, while foods that contain carbohydrates that decompose more slowly tend to have a low IG. The concept was developed by Jenkins and colleagues in 1980-1981 at the University of Toronto for their research on the optimal diet for patients with diabetes []. Regular consumption of high GI foods, compared to isoenergy meals and controlled by low GI nutrients, results in levels of glucose and insulin of blood greater than 24 hours, higher C-peptide excretion and higher HbA1c concentrations in non-diabetic and diabetic individuals [, ].The post-hydrate absorption rate, after a meal High IG meals produce an initial period of glucose and high insulin levels, followed in many individuals by reactive hypoglyemia []. On the other hand, a low GI diet can improve diabetes management by reducing early postprandial hyperglycemia and lowering the risk of postabsorptive hypoglycemia []. After a low GI meal, hypoglycemia and hormonal sequel do not occur during the postprandial period due to the continued absorption of nutrients from the gastrointestinal tract and the growing production of liver glucose. The consumption of foods containing identical energy and nutrients can produce markedly different physiological responses []. Therefore, low gas consumption meals would be the meals of choice among those that had reactive hypoglycemia. It has been shown that a low GI meal resulted in significantly lower plasma glucose, serum insulin and LPG-1 plasma than high GI food []. Another study examined the short-term effects of a low or high simple GI food on the plasma levels of LPG-1, PYY, and insulin in twelve patients []. A medium-sized GI dinner was provided, and participants fasted during the night, and a low or high breakfast of GI was consumed in the morning and blood was taken every 30 minutes for 150 minutes. This intervention was repeated with a test meal after a 2-week wash period. There were significantly lower insulin levels after low GI breakfast compared to high GI breakfast []. Thus, a low GI diet can be therapeutic for individuals with reactive hypoglycemia. In addition, low-level GI diets are now recommended in the evidence-based nutrition guidelines for diabetes prevention and management (DVG, 2011) []. In addition to the glucaemic index, the glucaemic load also needs consideration. The research has shown that low glucose load foods were associated with a lower insulin, glucose, and urge to eat grades but with a higher ghrelina []. In view of the above evidence, both low GI diets and low glucose loads are the main pillar of patients experiencing reactive hypoglycemia. ReferencesCopyrightCopyright © 2013 Kevin Stuart et al. This is an open access article distributed under the article, which allows unrestricted use, distribution and reproduction in any medium, provided that the original work is duly cited. More related articles Share Related articles
Glucose Tolerance Test Glucose TolerancePurposedetermine how quickly blood glucose is cleaned The glucose tolerance test is one in which it is given and the samples taken afterwards to determine how fast it is cleaned from the blood. The test is usually used to test for , , impaired function, and sometimes and , or rarer disorders of . In the most common version of the test, an oral glucose tolerance test (OGTT), a standard dose of glucose is ingested by mouth and blood levels are reviewed two hours later. Many variations of the GTT have been designed over the years for various purposes, with different standard doses of glucose, different administration routes, different intervals and sampling duration, and various measured substances in addition to blood glucose. glucose tolerance testContent History[] The glucose tolerance test was first described in 1923 by . The test was based on the previous work in 1913 by A. T. B. Jacobson to determine that carbohydrate ingestion results in blood glucose fluctuations, and the premise (called after its first H. Staub observers in 1921 and K. Traugott in 1922) that a normal glucose-fed patient will quickly return to normal blood glucose levels after an improved initial peak, and then see glucose. Testing[]Since the 1970s, organizations interested in diabetes agreed on a standard dose and duration. Preparation[] The patient is instructed not to restrict intake in the days or weeks before the test.[] The test should not be done during a disease, as the results may not reflect the metabolism of the patient's glucose when it is healthy. A complete dose of adults should not be given to a person who weighs less than 42.6 kg (94 lb), or excessive glucose may produce a result. Generally, the OGTT is performed in the morning as glucose tolerance can show a day rhythm with a significant decrease in the afternoon. The patient is instructed (water is allowed) for 8 to 12 hours before testing. Medication as large doses of , , and affect glucose tolerance test. Procedure[]Glucose and variations are suspected[] Measures and variations[] ]If you are suspected of excreted sugar in the urine despite normal blood levels, urine samples can also be collected to perform tests along with fasting and 2-hour blood tests. Results[]Pulsion of plasma glucose2 hour GTTFor , the (ACOG) recommends a two-step procedure, where the first step is a dose of glucose of 50 g. If after 1 hour the blood glucose level is more than 7.8 mmol/L (140 mg/dL), it is followed by a dose of glucose of 100 g. Diagnosis of gestational diabetes is then defined by a blood glucose level meeting or by overcoming cutting values at least two intervals, with cuts as follows: Sample method[]The diagnostic criteria indicated above by the World Health Organization (WHO) are only for venous samples (a sample of blood taken from a vein in the arm). An increasingly popular method to measure blood glucose is the sample of capillary or puntiagued blood, which is less invasive, more convenient for the patient and requires minimal training to drive. Although glucose levels in fasting blood have been shown similar in capillary and venous samples, the glucose levels in postprandial blood (measured after a meal) may vary. Diagnostic criteria issued by WHO are only suitable for venous blood samples. Given the growing popularity of capillary testing, WHO has recommended that a conversion factor between the two types of sample be calculated, but since 2017 no conversion factor had been issued by WHO, despite the fact that some medical professionals adopt their own factor. Variations[ A standard two-hour TG (glucose tolerance test) is sufficient to diagnose or exclude all forms of diabetes mellitus at all, but the first stages of development. Longer tests have been used for a variety of other purposes, such as detecting reactive hypoglyemia or defining subsets of hypothalamic obesity. Insulin levels are sometimes measured to detect insulin resistance or deficiency. GTT (glucose tolerance test) has a limited value in the diagnosis of reactive hypoglycemia, since normal levels do not exclude diagnosis, abnormal levels do not prove that the other symptoms of the patient are related to a proven atypical OGTT, and many people without symptoms of reactive hypoglycemia may have late low glucose. Oral glucose challenge test[]The oral glucose challenge test (OGCT) is a short version of the OGTT, used to check pregnant women to detect signs of . It can be done at any time of the day, not in an empty stomach. The test involves 50 g of glucose, with a reading after an hour. Limitations of OGTT[ ]The OGTT does not distinguish between insulin resistance in peripheral tissues and the reduced capacity of the pancreatic beta cells to produce insulin. The OGTT is less accurate than the (the "golden standard" to measure insulin resistance), or the , but is technically less difficult. None of the two technically demanding tests can be easily applied in a clinical environment or used in epidemiological studies. HOMA-IR () is a convenient way to measure insulin resistance in normal subjects, which can be used in epidemiological studies, but can give misperforming results for diabetic patients. ######################## ################################################################################################################################################################################################################################## Other Navigation menu Personal tools Named spaces Variants Views More Search Navigation Contributed Tools Printing/exporting Languages

Oral Glucose Tolerance Test: Indications and Limitations - Mayo Clinic Proceedings
Acute hypoglycemic effect and oral glucose tolerance test (OGTT) in... | Download Scientific Diagram![PDF] Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? | Semantic Scholar PDF] Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/146cedc10661a4789671dcf545fd75b00968b0f3/4-Table3-1.png)
PDF] Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? | Semantic Scholar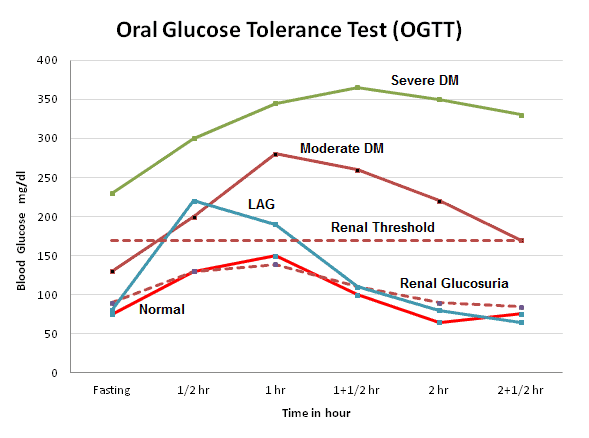
Glucose Tolerance Test (GTT) : Principle, Procedure, Indications and Interpretation | LaboratoryInfo.com
how low? | Final Trick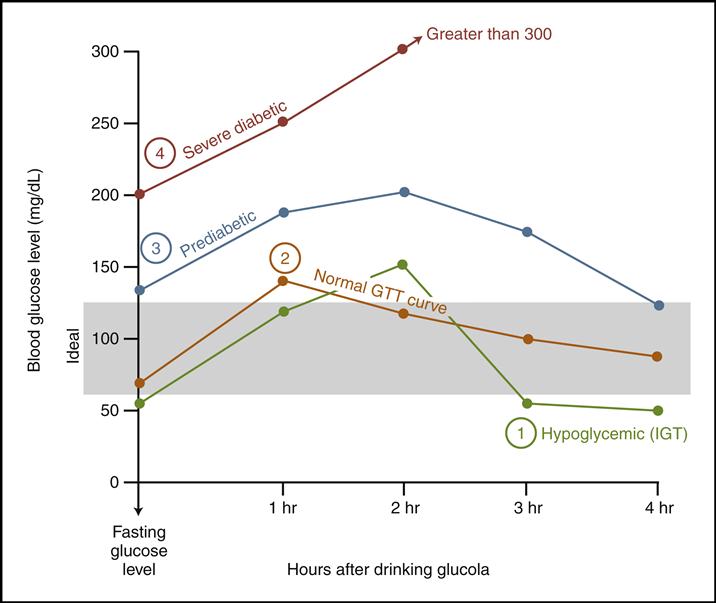
Chapter 4 Details | Metabolic Insight
Hypoglycemic Health Association of Australia - Testing For Hypoglycemia And How Your Doctor Can Help
Postprandial Reactive Hypoglycaemia: Varying Presentation Patterns on Extended Glucose Tolerance Tests and Possible Therapeutic Approaches
Figure 4 from VARIANTS OF ORAL GLUCOSE TOLERANCE TEST (OGTT) CURVE IN GESTATIONAL DIABETES MELLITUS (GDM) | Semantic Scholar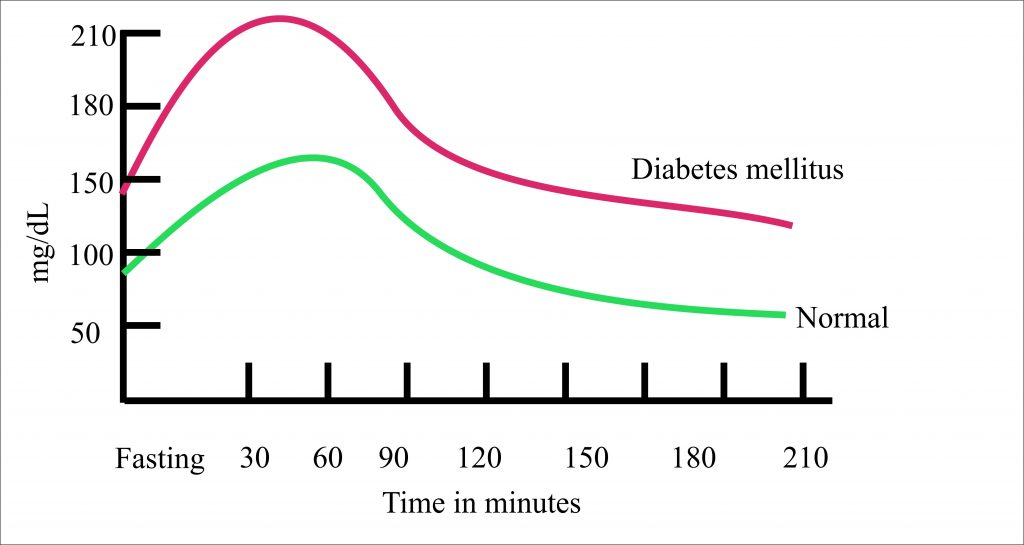
Diabetes Mellitus:- Part 4 – Gestational Diabetes Mellitus, Oral glucose tolerance test, (OGTT) – Labpedia.net
Hypoglycemia-Medical Marijuana Treatment
Oral Glucose Tolerance Test Revealed Reactive Hypoglycemia With... | Download Table
Abnormal glucose tolerance testing following gastric bypass demonstrates reactive hypoglycemia | Semantic Scholar
Low Blood Sugar - Hypoglycemia - Affects Health, Emotions and Learning Ability/1087684_color-5b9ab19546e0fb00258fae9d.png)
Oral Glucose Tolerance Test Uses, Procedure and Results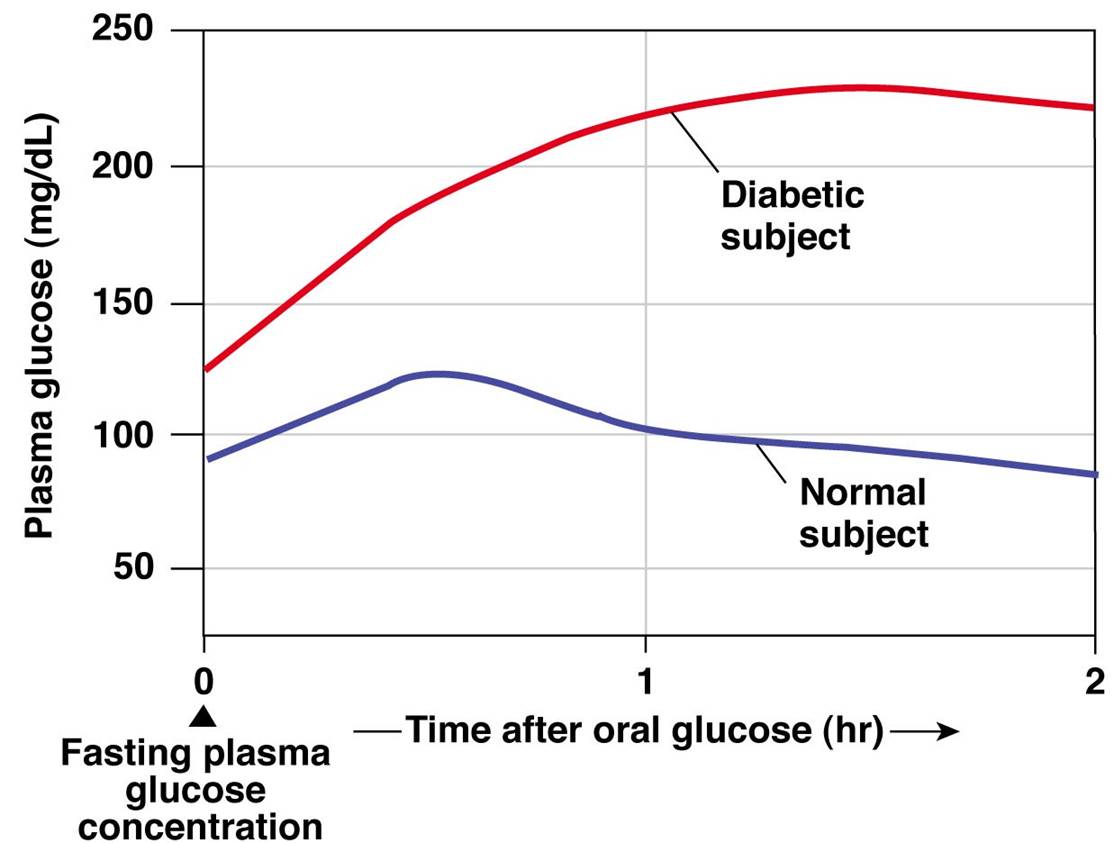
Glucose Regulation
Hypoglycemia and aggression
PDF) Prolonged Oral Glucose Tolerance Test in Non-Diabetic Patients with Ethanol Poisoning
Secrets of Oral Glucose Tolerances Test (OGTT, GGT ) Type and Normal Value and curve - YouTube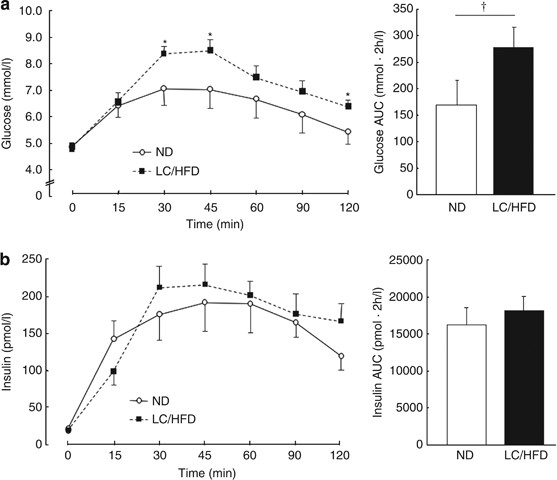
Short-term low carbohydrate/high-fat diet intake increases postprandial plasma glucose and glucagon-like peptide-1 levels during an oral glucose tolerance test in healthy men | European Journal of Clinical Nutrition
Hypoglycemia Glucose Test![Almond]()
Almond "Appetizer" Effect on Glucose Tolerance Test (GTT) Results | American Board of Family Medicine
Evaluation of hypoglycemic and hypolipidemic activities of aqueous extract of Cistus ladaniferus in streptozotocin-induced diabetic rats - ScienceDirect![PDF] Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? | Semantic Scholar PDF] Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/146cedc10661a4789671dcf545fd75b00968b0f3/3-Table2-1.png)
PDF] Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? | Semantic Scholar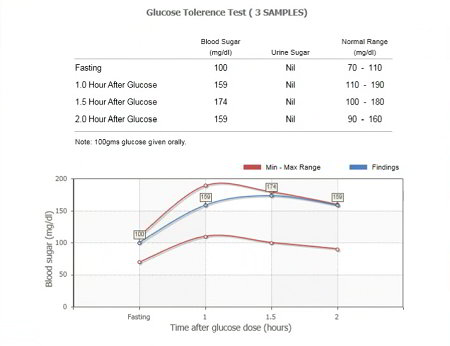
Hypoglycemia - Symptoms, Diagnosis and Treatment
Assessing the Shape of the Glucose Curve During an Oral Glucose Tolerance Test | Diabetes Care
Delay in glucose peak time during the oral glucose tolerance test as an indicator of insulin resistance and insulin secretion in type 2 diabetes patients - Wang - 2018 - Journal of
RACGP - Oral glucose tolerance testing
Effect of different periods of paradoxical sleep deprivation (PSD) on... | Download Scientific Diagram
Glucose Tolerance - an overview | ScienceDirect Topics
NERL™ Trutol™ Glucose Tolerance Test Beverages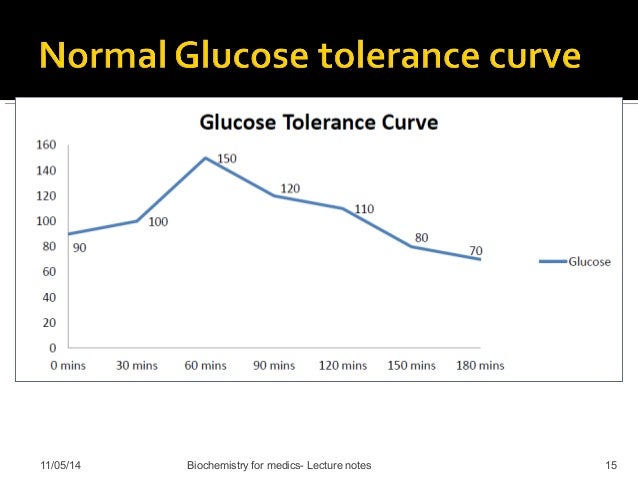
Glucose Tolerance Test
Glucose Intolerance - Signs, Symptoms, Treatment and Diet
Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes?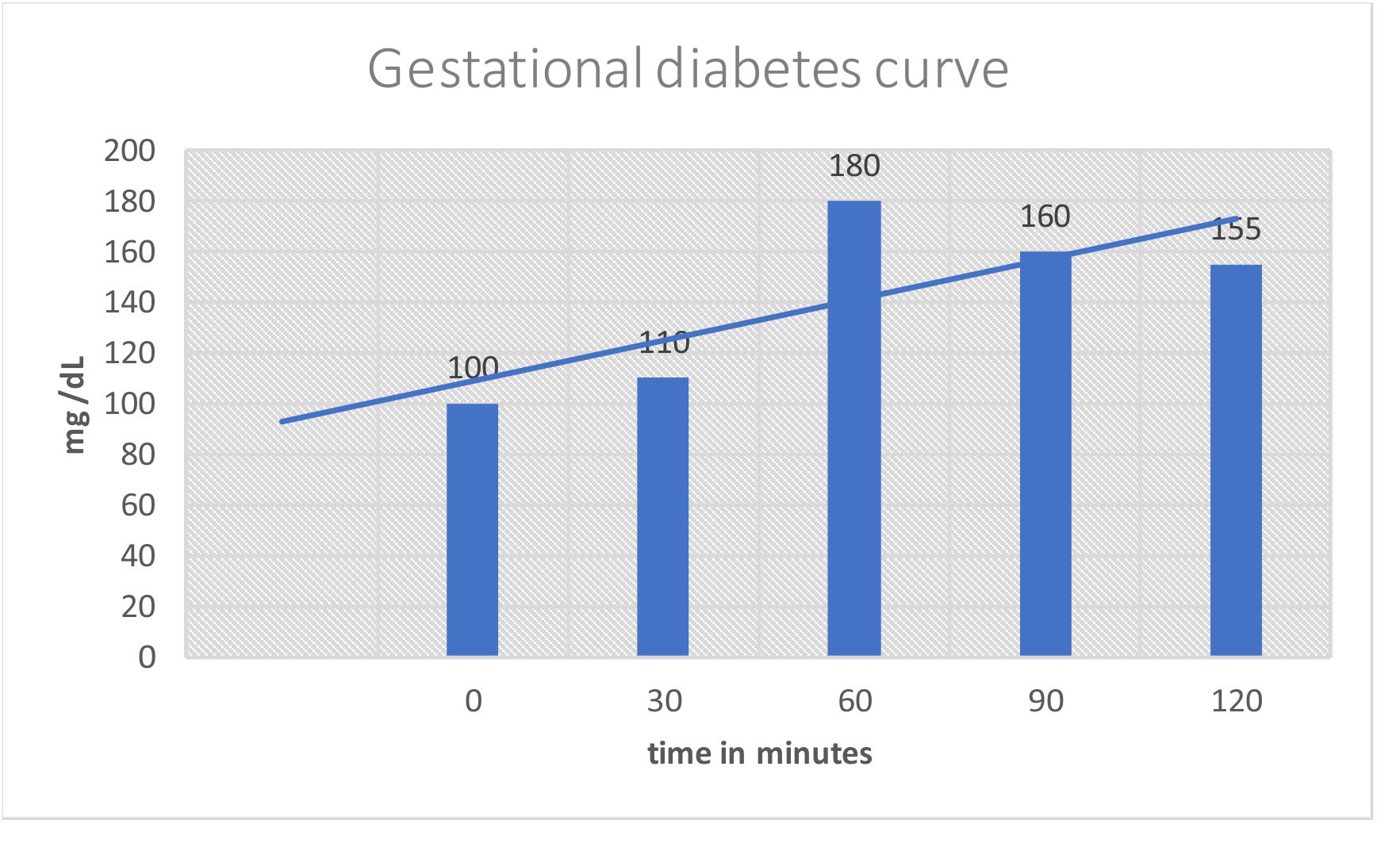
Diabetes Mellitus:- Part 4 – Gestational Diabetes Mellitus, Oral glucose tolerance test, (OGTT) – Labpedia.net/what-to-know-about-reactive-hypoglycemia-1087744-ADD-FINAL-V2-816aeb42a8454caca819e5eb00f77d49.png)
Reactive Hypoglycemia: Overview and More
Oral Glucose Tolerance Test: Indications and Limitations - Mayo Clinic Proceedings
Oral glucose tolerance test results for glucose (a) and insulin (b).... | Download Scientific Diagram
JCM | Free Full-Text | The Oral Glucose Tolerance Test—Is It Time for a Change?—A Literature Review with an Emphasis on Pregnancy | HTML
Figure 1 from Successful treatment of reactive hypoglycemia secondary to late dumping syndrome using miglitol. | Semantic Scholar
 Hypoglycemia during oral glucose tolerance test among post–bariatric surgery pregnant patients: incidence and perinatal significance - ScienceDirect
Hypoglycemia during oral glucose tolerance test among post–bariatric surgery pregnant patients: incidence and perinatal significance - ScienceDirect

![PDF] Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? | Semantic Scholar PDF] Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/146cedc10661a4789671dcf545fd75b00968b0f3/4-Table3-1.png)









/1087684_color-5b9ab19546e0fb00258fae9d.png)






![PDF] Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? | Semantic Scholar PDF] Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/146cedc10661a4789671dcf545fd75b00968b0f3/3-Table2-1.png)










/what-to-know-about-reactive-hypoglycemia-1087744-ADD-FINAL-V2-816aeb42a8454caca819e5eb00f77d49.png)




Posting Komentar untuk "hypoglycemia after glucose tolerance test"